Abstract
Introduction
HHV-6 reactivation has been associated with transplant complications such as graft failure, acute GVHD, and CMV reactivation. However, the causal relationships and the involved mechanisms remain largely speculative. In addition, as the immunosuppressive settings that promote HHV-6 reactivation may also increase the risk of reactivation of other viruses (e.g. CMV), determining the independent effect of HHV-6 is difficult. In this retrospective study, we focused on early reactivations (until day 30 post-transplant) and their impact on relapse because of the well-established immunosuppressive effect of HHV-6 on the innate and adaptive immune system, which could theoretically weaken the graft-versus-tumor (GvT) effect, resulting in more relapse and worse survival.
Methods
To eliminate potential confounders and address the independent effect of HHV-6 reactivation, we restricted our population to CMV-seronegative recipients of UCB (2011-2017) and excluded those with DNA virus reactivations other than HHV-6 (CMV, adenovirus, EBV, and BK virus). HHV-6 reactivation was defined as ≥1 positive quantitative DNA PCR on whole blood within 30 days post-transplant. The absence of HHV-6 reactivation was defined as ≥1 negative PCR in the first 2 weeks and ≥1 negative PCR in the second 2 weeks post-transplant. A subset of patients had research blood samples collected on day 30, which we used to measure functionally relevant T-cell subsets by flow cytometry.
Results
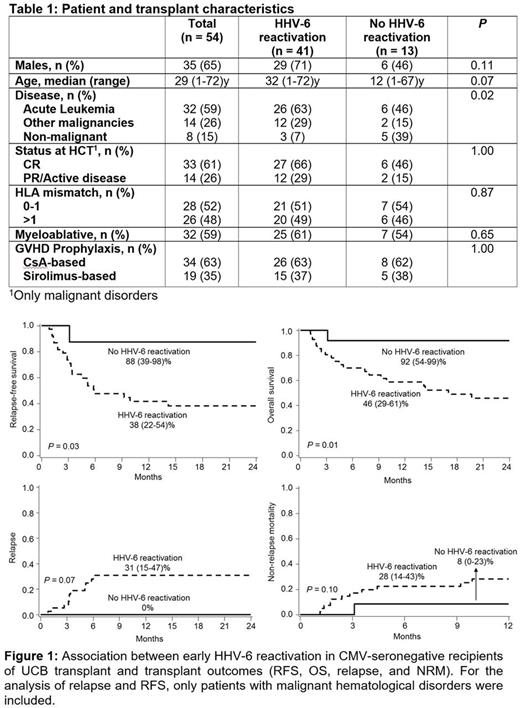
54 patients were included (41 with and 13 without HHV-6 reactivation; Table 1). The groups were similar except more malignant disorders in those with HHV-6 reactivation, P= 0.02). The HHV-6 reactivation group had a lower RFS (38 [22-54]% vs. 88 [39-98]%, P= 0.03; Figure 1A), lower OS (46 [29-61]% vs. 92 [54-99]%, P= 0.01; Figure 1B), and more relapse (31 [15-47]% vs. 0%, P= 0.07; Figure 1C) at 2 years plus a trend for higher 2-year NRM (28 [14-43]% vs. 8 [0-23]%, P= 0.10; Figure 1D). For the latter two results, only patients with malignant hematological disorders were included. Neutrophil recovery, graft failure, acute GVHD grade II-IV, and chronic GVHD were similar in both groups.
Next, we evaluated day 30 blood T-cell immunophenotype focusing on 3 markers with potential relevance to T-cell function: (i) CD57 (associated with T-cell replicative senescence), (ii) NKG2a (associated with resistance to immunotherapy in solid tumors), and (iii) KIR2DL2/3 (known to prematurely end the immune synapse and inhibit sustained TCR-induced gene transcription resulting in decreased IFN-γ production). The relative abundances of T cells expressing CD57, NKG2a,or KIR2KL2/3 were all significantly higher in the group with HHV-6 reactivation (P < 0.05). Interestingly, the abundance of NKG2c+CD57+ cells among NKG2a+ NK cells (so called "adaptive" NK cells) was marginally significantly higher in the group with HHV-6 reactivation (P= 0.07).
Conclusions
Previous studies have reported higher NRM in patients who reactivate more DNA viruses post-transplant (Hill J, et al. Blood 2017). We demonstrated an independent association between early HHV-6 reactivation and worse RFS/OS, attributed in part to relapse. This was consistent with the observed skewed T-cell repertoire in the group with HHV-6 reactivation, which showed markers of decreased immune effector function, possibly reflecting an attenuated GvT effect. Although the relative abundance of adaptive NK cells was somewhat higher in the group with HHV-6 reactivation (reminiscent of CMV reactivation), this did not seem to rescue the GvT effect which was apparently impaired due to T-cell defects. If confirmed in larger cohorts, rigorous prophylaxis and/or HHV-6 vaccination may be explored to decrease the risk of relapse and improve survival.
Miller: Celegene: Consultancy; Oxis Biotech: Consultancy; Fate Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal